Diamond: Magic and Medical Properties. Almaz stone - a combination of beauty, magic and incredible strength
Properties and description of the stone "Almaz"
Diamond - This is the most popular, most precious stone in the world. The diamond is a diamond in the treated form. It is believed that the name "Diamond" originated from the Greek word Adamas is an irrevocable, disadvantageous. Founded diamonds are called diamonds. This name happened from french word. Brilliant that translated into Russian means "sparkling". Usually it is colorless or has weak yellow, brown, gray, green, sometimes pink shades, rarely - black. There are about 1000 varieties of jewelry diamonds. Diamond is a symbol of hardness, courage, innocence. This stone gives the owner hardness and courage, protects members of his body. It was believed that the diamond gives and retains the sharpness of hearing, protects against sorrow and witchcraft, evil spirits. If some sorcerer wants to get the diamond owner, then all the grief and failures will be transferred to him. The wild beast does not attack a person who carries a diamond. Stone can cure from Lunaticism. Diamond must be purchased freely, without coercion and violence, then he will have a great power. The stone will lose force due to sinfulness, the impotence of a person who is wearing. Diamonds, who are inherited from the mother - daughters, are of great strength, and son. If you bought them, "work" on you will not start immediately.
Diamonds with greenish tump strengthen the ability of a woman to childbear, help the normal course of pregnancy and facilitate childbirth. They treat sclerosis, apoplexy, prevent the formation of stones, soothe migraine, save from lightning shocks and evil eye.
Diamond is associated with the energy of the Sun and is used as a heart tonic. According to the convictions of the ancients, this stone effectively rejuvenates and provides spiritual development. If you wear a ring with a diamond in gold on a ring finger right, it will allow you to feel the subtle effect of energy on your body. You can not wear other challenge rings.
Colorless diamonds are rare. Most often they have any shade. In nature there are diamonds, brightly painted in yellow, orange, green, blue, blue, pink, brown, gray and black.
To refer to the color of the diamond, there is the following gradation, including 12 colors.
- Jater - bluish white;
- River - absolutely transparent bluish-white;
- Tone Visselton - pure and white, less transparent;
- Visselton - white;
- Top Crystal - with a light shade of yellow color;
- Kristl - with a slightly more noticeable shade of yellow;
- Verp Light - with a very small shade of brown;
- Top Cape - slightly yellowish;
- Cape. - yellowish;
- Light Yello. - light yellow;
- Light Brown - light brown;
- Yello. - Yellow.
Chemical composition of Almaz
Mineral, crystalline polymorphic modification of native carbon, glitter, beauty and hardness superior all minerals. Diamond consists of approximately 96-99.8% of carbon, 0.2-0.3% are impurities chemical elements, such as nitrogen, oxygen, aluminum, boron, silicon, manganese, copper, iron, nickel, titanium, zinc, etc.
Physico-chemical properties of diamond
a) Formula: C;
b) admixture: n;
c) Singonia: cubic;
d) color: colorless, yellowish, brown, sometimes green, blue, reddish, black;
e) color features: absent;
e) shine: diamond;
g) transparency: transparent;
h) hardness: 10;
i) Spoundism: Perfect software (111);
K) Fravel: sink to a snapshot;
l) density: 3.47-3.55 g / cm 3;
m) refractive index: 2.417-2,419.
Coloring distribution is often uneven, spotted or zonal. Under the action of X-ray, cathode and ultraviolet rays, most diamonds begin to glow (lumine) blue, green, pink and other flowers.
The diamond sticks to some fatty mixtures, this was based on the greatest fat method of extracting diamonds at the processing factories. On the air, the diamond burns at 85 o C with the formation of CO 2; In vacuum at temperatures above 150 ° C passes into graphite.
Features of the formation of diamond
 It is assumed that the diamond is crystallized by one of the first minerals when the mantle silicate melt is cooled at a depth of 150-200 km at a pressure of 5000 MPa, and then removed to the surface of the Earth as a result of explosive processes accompanying the formation of kimberlite tubes, 15-20% of which contains diamond.
It is assumed that the diamond is crystallized by one of the first minerals when the mantle silicate melt is cooled at a depth of 150-200 km at a pressure of 5000 MPa, and then removed to the surface of the Earth as a result of explosive processes accompanying the formation of kimberlite tubes, 15-20% of which contains diamond.
Diamonds are also found in the deep breeds of eclogs and some deep-albertic grenade gneisses. Small diamonds in significant amounts were found in meteorites, as well as in gigantic meteorite craters, where composhable rocks contain significant amounts of fine-crystalline diamond.
The dimensions of the crystals range from microscopic to very large, the mass of the largest diamond "Kullynan" found in 1905 in South Africa 3106 car (0.621kg).
Deposit Almaz
Industrial deposits are associated with kimberlites, placers. Chief foreign mining country: South Africa, Congo (Zaire), Botswana, Namibia. IN Russian Federation Deposit in Yakutia, in the Urals.
Application Almaz.
Diamond is applied in industry as an abrasive material, as well as, of course, in jewelry.
But, without cut diamond, it looks not very attractive. The surface of crystals produced from the Earth in most cases is rough and coated with a translucent fascular gray crust.
Just polished and restroing the diamond in a diamond, the person saw that "this is the light of the sun, thickened in the ground and chilled by time, he plays all the colors of the rainbow, but he himself remains transparent, like a drop." So spoke about Diamond Russian writer A.I. Kuprin.
Therapeutic properties of Almaz
In ancient times, people understood that the diamond heals his owner from mental disorders: different phobias, depression, nervous disruptions. Lithotherapists say that the stone adjusts the nervous system of man and sets it on a healthy lifestyle. Therefore, they recommend to take advantage of this ability to get rid of alcoholism, uncontrolled drunkenness, tobacco and further to treat drug addiction. In addition, the diamond is considered an excellent antipyretic agent, a means for the treatment of inflammation of lungs, bronchitis, prostate gland, hepatitis, various infections and diseases of the joints.
Magic properties of Almaz
Diamond is the most valuable, but also the most dangerous stone for a person. However, this does not mean that he harms a person, simply, if it is rude and impolite about him, he can harm the owner.
For a long time, it is believed that the diamond gives her owner courage, determination, spirituality of the spirit, brings success, protects against alcoholism, drug addiction, promotion, tightens excessive fantasies, eliminates the seals, dismisses the witchcraft chairs, protects against evil eyes. However, it should be remembered that diamond is a stone of kings, high priests and celebrities. He serves high-ranking personalities faithfully, to ordinary people, this proud stone is indifferent. But if you got him inheritance or as a gift, he will help you, only this will happen quite soon - after 7-10 years. All this time, the diamond will be tuned on the new owner and, to understand its essence, will begin to work actively on it.
Diamond is the first zodiac stone, since it has a huge energy force. He reflects the whole zodiac and is the "leader" of all other minerals.
This stone must have every Aries, since the diamond tamens anger, hot temper, irritability. However, he is not contraindicated by the remaining signs of the zodiac.
Talismans and amulets from diamond
As a talisman, Diamond can be worn on the nameless finger of the left hand, as well as in the form of a serylet or a snap, so that the stone concerned the skin. It will also help in heartfers, and in work, but with one condition - the intentions of a person should be honest and moral. Thieves, murderers, fraudsters and swells he will bring some misfortunes.
It is known that one of the first major diamonds is the famous "Star of South Africa" \u200b\u200bweighing 83.5 carats - was redeemed from the sign - a sorcerer who used him during spells.
Jewelry diamonds
Jewelry diamonds usually account for 20-25% mined diamonds; In the marginal deposits, their share is noticeably higher than in the indigenous. Jewelry diamonds are transparent, without crack inclusions.
With their cut, the largest shine and the game of the stone are revealed, natural defects are eliminated, and about 50% of the initial mass is lost. A special diamond shape of the cut has been developed, but the cut is also used with wedges, cabochone, stepped and combinations thereof.
Mining and price for diamonds
 The annual global mining of diamonds is estimated at 100 million carries (20 tons), of which 40 million falls on countries in Africa and 30 million to Russia and Australia.
The annual global mining of diamonds is estimated at 100 million carries (20 tons), of which 40 million falls on countries in Africa and 30 million to Russia and Australia.
The world's largest monopolist for the extraction and sale of diamonds is the company "De Birs" with an annual turnover of more than 15 billion dollars. In Russia, the production of diamonds is maintained by the company "Diamonds of Russia - Sakha" (production volume in value terms is 1.3 billion dollars per year).
The mass of the mined diamonds is usually 0.1-1.0 car, large jewelry stones (St. 100 kars) are rare. The price of unprocessed diamonds of jewelry quality is about $ 100 for the car., The cost of diamonds is greatly fluctuated in the dependence of the purity of "water", the shades of the color of the "blooming", the presence of inclusions and defects, the size of the stones, the quality of the cut, etc. The usual price of the carat of diamonds - from 400 to 1000 dollars.
Technical diamonds are used in diamond drill crowns, saws, cutters, filters for extrusion wire, for the manufacture of polishing powders and pastes, as well as in the optical and electronics industry as semiconductors.
DIAMOND STONE FACTS, INFORMATION DESCRIPTION AND PROPERTIES
Their diamond. IS NOT ONLY THE HARDEST GEMSTONE, IT IS IN FACT THE HARDEST MINERAL ON EARTH. That Combined with It's Brilliant Fire, And Exceptional Lust Has Made The Diamond One of the World of Prized Gemstones in the world.
Pure, Colorless Diamonds Are The Most Popular, But Fancy Colored Diamonds Are Quickly Becoming Quite Popular ThemSelves! From Yellow and Brown to Green, Blue, Pink, Red, Gray and Black, Can All Be Found Depending on the Impurities present.
Fancy Colored Diamonds Are Not a Mass-Market Product That Are Advertised Everywhere and Sold in Large Numbers. They Have Much More Personality Than that. Fancy Colored Diamonds Are Almost AS Much Fun As Colored Gemstones! Like Colored Gemstones, Each One Is Different.
SCIENTFIC PROPERTIES OF DIAMOND
MOHS HARDNESS OF 10 WITH A CUBIC CRYSTAL STRUCTURE.
Diamonds Are Graded by The Four C's: Cut, Color, Clarity and Carat (Weight).
Diamonds Form At High Temperatures and Pressures More Than 50 Miles Underground. Although South Africa Became The Highest Producer Of Diamonds In 1870, Australia Is Currently The Main Producer, with other Locales Including Ghana, Sierra Leone, Zaire, Botswana, The US and Brazil.
Mystical Properties of Diamond
In Ancient Times, Diamonds Were Worn AS Tumbled Stones. THEY WERE TREASURED FOR THEIR BEAUTY AS A Gemstone. IT Was Not Until Most Recently After Creating A Facet Was Discovered Than Their Dazzling Personality Truly Appeared.
The Diamond Is A Stone That Bonds Relationships and Enhances Love-With The Most Recent Symbol of the Engagement Ring. There Is Little To No History That It Is A Stone Of Love.
What the Diamond Does Bring Is Longevity, Particularly to Relationships, Balance, Clarity and Abundance. IT CAN AMPLIFY ONE'S THOUGHTS, WHETHER THEY BE STRENGTHS, OR WEAKNESSESS. IT Gives One WEARS OR CARRIES IT COURGE AND HOPE.
A Diamond Encourages Self-Confidence And Desire for Independence While At The Same Time Counteracting Jealousy.
Healing Properties of Diamond
The Diamond Is Known As a Master Healer, It Breaks Up Blockages in Both The Crown Chakra and The Personality.
Historically, Crushed Diamond Has Been Used As A Cure for Many Ailments. A Small Piece of Rough Diamond Placed Overnight in A Glass of Water to Be Durunk During The Following Morning, Has Been Know AS An Excellent Fortifying Remedy for Combating Stress; Especially During Periods of Exhaustion and Convalescence.
Care Must Be Used When Wearing A Diamond Necklace As It Can Block The Energy Flow If The Wearer Has Negative Thoughts or Feelings.
Magical Properties of Diamond
- ENERGY: Projective.
- Element: Fire.
- Powers: Spirituality, Sexual Dysfunction, Protection, Courage, Peace, Reconciliation, Healing and Strength.
The Diamond Has A Wide and Varied Magical Repertoire. WORN AS Jewelry Is IS Known to Promote Spirituality, Even Ecstasy, The Shaman's Ritual State of Consciousness. IT IS COMMONLY USED IN MEDITATION AND PURSUITS OF SPIRITUALITY.
BECAUSE OF ITS HARDNESS AND IT'S COMMON ASSOCIATIONS WITH THE SUN, THE DIAMOND IS OFTEN WORN OR UTILIZED IN SPELS TO INCREASE PHYSICAL STRENGTH. In Ancient Rome, It Was Sent in Steel Rings Created So That The Stone Would Physically Touch The Skin. The Helped The Soldiers Produce More Bravery, Daring and Eventual Victory.
A Diamond Is Considered A Magical Stone Of Great Power. IT IS Said to Enhance All The Energies in The Body, Mind and Spirit, Thus Helping With Alignment with the Higher Self. The Diamond, Owing to IT's Natural Flashy Nature, Has Long Been Known As A Stone of Protection. Having It Faceted Into A Six SiDed Cut Is The Best Assurance That It Will Bring The Wearer Lifelong Luck.
Try scrying with a Faceted Diamond in Soft Candle Light, And Delight Yourself with Its Exquisite World of Color and Light. Please Remember That Diamonds Should Not Be Cleansed or Charged.
Zodiac
- Planets: Sun.
Traditional Birthstone for The Monteh of April.
Stone Of Leo, The Diamond Provides Enlightenment, Purity and Clarity. IT Provides Protection, Preserves Peace and Gives Power.
Chakra Classification
Diamond Is Associated with the 7th, or Crown Chakra.
The Diamond Has a Harmonizing Influence On All Of The Chakras. Placed on the "Third Eye", A Diamond Will Help Combat Mental Illness, by Activating and Encouraging The Third Eye.
Fans of precious stones are very interesting about the structure of the structure of diamond, the description of its and basic physical, mechanical and chemical properties. This beautiful stone in its chemical structure Refers to nonmetallam and has a crystal structure. Speaking by the language of chemists, Adamant is a cubic altropy carbon form. In jewelry art, this form of carbon is considered the most expensive of precious stones, and decorations with Adamant are very expensive. This is due to the fact that the glitter of the crystals of this substance is impossible to compare anything. And besides, he will not fade and does not scratch. That is, the polished surface of crystals in the jewelry is always pleased with the eye.
No matter how paradoxically sounds, but Adamant and Graphite have the same structure. And these two such diametrically opposite substances have one nature. The fact is that the diamond and graphite are formed by carbon atoms. Consider the structure and properties of the diamond.
According to the structure, the diamond crystal has the shape of the tetrahedron, and at the same time carbon atoms are located in the center. The tops in such a tetrahedra serve as the most close-based carbon atoms. A very stable atomic connection is obtained in the structure of the crystal, and this explains the increased strength of the substance. Between the atoms from which the elementary cell is linked to a covalent bond. This feature explains the high density of diamond.
 In general, the diamond crystal can be represented as a molecule of giant sizes. Recall that the molar mass of this crystal is 12. The form of the crystal is not associated with the number of faces at the jewelry stone. The edges of the diamond appear when processing it.
In general, the diamond crystal can be represented as a molecule of giant sizes. Recall that the molar mass of this crystal is 12. The form of the crystal is not associated with the number of faces at the jewelry stone. The edges of the diamond appear when processing it.
According to the chemical structure, diamond is pure carbon. But its composition still includes impurities. Conducted chemical analysis made it possible to determine the presence of a certain number of other substances. The impurities include substances such as:
- nitrogen;
- magnesium;
- aluminum;
- silicon.
And many other chemical elements of the Mendeleev table. Moreover, many of the elements are isomorphic inclusions. But people use diamonds not only for making jewelry. Wide use received this crystal in the technique. And all this thanks to her unique properties and the highest strength.
The video presented well shows the crystal structure of the diamond.
Physical properties of Almaz
Diamond is the hardest substance that is found in nature.
One of the types of Adamanta - Corundum - has a similar structure, but a low hardness (Corund's hardness is lower than that of Adamant 150 times). It is necessary that the hardness of the substances is determined on the MOOS scale. On this rankire, the diamond is assigned the highest hardness indicator - 10.
 It would be possible to be used to treat metals, including high-strength, and solid minerals, such as beryl, grenades, sapphire and others. Diamond tools are very resistant to abrasion. The hardness and density of diamond is higher than that of quartz and corundum.
It would be possible to be used to treat metals, including high-strength, and solid minerals, such as beryl, grenades, sapphire and others. Diamond tools are very resistant to abrasion. The hardness and density of diamond is higher than that of quartz and corundum.
But with all the hardness of the diamond, high fragility. And even a high density pronounced does not reduce the likelihood of split when falling. After all, pure crystalline carbon, which is diamond, has a multilayer structure. And with sharp blows about a solid surface, it is possible to split in those places of the structure, where the connection between atoms is very weak. It is in places of spike of atoms and a split occurs.
And with all the durability and durability of this substance, it needs to be removed from drops on a solid surface. This variety of carbon and the highest thermal conductivity among all solids. The thermal conductivity of the diamond is from 20 to 24 W / cm. It should also be said that the diamond is a dielectric. This is due to the peculiarities of atomic ties in the crystal of this substance.
The temperature of the combustion of diamond in oxygen is 800 ° C. This type of carbon is burning a beautiful blue flame. But at a temperature of 2000 ° C and in the absence of oxygen, this beautiful mineral turns into graphite. Indicators of melting point in diamond are equal to 3700-4000 ° C.
The most basic and valuable property of a diamond is its refractive index and a high degree of dispersion. The brilliance of diamonds depends on these characteristics and is a distinctive feature of this precious mineral. The weight of the diamonds is measured in carats. At the same time, the weight of one carat of diamond is about 0.2 grams. To determine this magnitude, jewelers have the necessary tables and information.
Diamond is the most durable, the most brilliant and most desirable stone in the world. How many status attributes the perfectly cut stone to its owner, how many admiring views, how much the heroines of books, movies and secular chronicles have received avalanches of attention, putting into the light of diamond decorations!
Diamond is a natural mineral. By cutting, it is called a diamond and is used in jewelry.
There are about a dozen types of cuts, in different ways presented stone. The greater the diamond faces, the more difficult and more fancy through it the rays of the light and give more shine. The initial diamond looks muddy and opaque.
History and interesting facts
Ancient Roman philosopher and statesman Guy Pliny Senior in its work "Natural History" emphasizes the uniqueness and value of the diamond. The highest price is not only among the stones, but among human things, he says, has a diamond, a long time known only to the kings, and not everyone.
However, until the 16th century, the stones almost did not know how to limit, they heard only one part of the mineral, turning the stone into an octahedron and no more. Therefore, for a long time it was valuable, as the diamond looks like and how beautiful it is, and as far as it is durable.
The name of the most famous gem stone occurred from the Arabic word "Almas" - the solidity and Greek "Adamos" - disgraceable. Therefore, there was now an obsolete name of the diamond - "Adamas".
From the 16th century, the technical capabilities of the cut appear, and the value of the diamonds increases sharply. Previously, diamonds were taken as talismans on the road, the royal people used them in their decorations and symbols of power.
Kullyann - the largest diamond
The largest diamond was found in 1905, weighed 3106 carats and was called "Kullanan" by the name of the owner of the mine, where he was mined. Collins were present in Kullyanne, so it was impossible to make a solid product from it. The stone was divided into two large parts and a few small. Seven years continued work on the cut, and at its end, the stone presented the British royal family.
The most expensive diamond was sold for nine million dollars at Sotheby's auction. In addition to the unconditional labor of the jeweler, in addition to the unconditional work, the jeweler includes the complexity of production, which occurs in several stages.
Diamonds are the most "bloody" stones from all gemstones. Due to their popularity and extremely high cost, they have become objects of killings and fraud throughout history.
Physical and chemical properties
Crystal cell
About the origin of diamonds does not exist unified opinion. The most common version is that the mineral formed in mountain departments, as a result of the cooled of substances in the robe of the earth's crust.
In nature, they are inclusions in graphics, can be in the river and marine coastal pebble plaques, to be located within the cooling of old volcanic rocks, where the lava occurred. Chemical diamond formula - pure carbon with minor enclosures of magnesium and iron. The crystal lattice has a cube shape.
Stone has an interesting feature: It shines with blue light with ultraviolet radiation directed.
Types of color
Absolutely colorless diamonds are rarely found, in the bulk they have colored shades of different intensity. Professionals appreciate bright colors with amazing game and color, and stone glitter.
Red Diamond - It is rare. Less than 1% of all diamond produced is orange. Most of them have different variations of yellow and brown shades. The classification rules define a diamond as orange if 25-50% orange is present in its color. These gems are mined in Austria and South Africa, and the cost for carat reaches forty thousand dollars. Orange diamonds carry a refreshing and soothing effect, stimulate creative successes.
 Black diamond appeared as a result of ultrahigh temperatures in terrestrial depths. These stones have one amazing property: Unlike other diamonds, they do not shine - absorb the color and do not reflect it due to the increased concentration of graphite at its base. In black diamonds, the uniformity of color distribution is valued. Such stones have powerful magical possibilities. They endow the owner by force and power, but also require energy equal to its.
Black diamond appeared as a result of ultrahigh temperatures in terrestrial depths. These stones have one amazing property: Unlike other diamonds, they do not shine - absorb the color and do not reflect it due to the increased concentration of graphite at its base. In black diamonds, the uniformity of color distribution is valued. Such stones have powerful magical possibilities. They endow the owner by force and power, but also require energy equal to its.
Red diamond is the most rare. Most jewelers did not even meet with such stones, and a very limited number of stones can be awarded the degree of truly red color. There is no proven opinion, why they appeared in nature. They are mined in Australia and in very small quantities in Brazil. Due to its exceptional uniqueness, 1 carat of pure red diamond can cost a million dollars. The stone is associated with the element of fire, with passion.
 Blue diamonds get their color from the presence of boron in their chemical structure. The first such stones were discovered in the 17th century in India, are now mined in South Africa and in Australia. Most often stones are not clean blue flowers, and shades with the impurities of green or gray. Depending on the brightness of the color and the degree of impurities are divided into 9 subgroups.
Blue diamonds get their color from the presence of boron in their chemical structure. The first such stones were discovered in the 17th century in India, are now mined in South Africa and in Australia. Most often stones are not clean blue flowers, and shades with the impurities of green or gray. Depending on the brightness of the color and the degree of impurities are divided into 9 subgroups.
Pink diamonds on the professional classification of hemologists are divided into four shades and 5 stages of color intensity. Mine in mines of India, Brazil and Australia. The largest pink diamond has a size of 186 carats.
Medical properties
Yoga note that the diamond comes very thin, but powerful energy capable of acting on the physical body, and on the psyche.
Stone has a beneficial effect on the nervous system: it is capable of fighting nightmares, fatigue, apathy and skin diseases. You can lower it into drinking water, insist and drink to promote health.
In Ayurveda, they talk about the anti-aging properties of the diamond. It reveals the chakras and configures on a special energy flow.
Magic properties
 The most efficient is the diamond transmitted by inheritance. He accumulates the generic memory and enhances its energy. In the East, it was believed that a purchased or presented new stone should fly in the house seven years before it can be started to wear.
The most efficient is the diamond transmitted by inheritance. He accumulates the generic memory and enhances its energy. In the East, it was believed that a purchased or presented new stone should fly in the house seven years before it can be started to wear.
However, any diamond has a powerful healing force and can enhance the effect of other stones. Strengthens all human energy centers, doubles contact and communicability. Magic properties The diamond interacts positively with the owner in the case of its non-violent acquisition, in the case of peace-loving thoughts of the host. Diamonds can not be worn by evil and dishonest people to avoid misfortunes.
Diamond is a stone of high-ranking persons. He serves as volitional and decisive individuals faithfully, to weak people indifferent. To wear diamonds stands with dignity and calm confidence in themselves, and then these noble stones will reveal on you in everything with their magnificence.
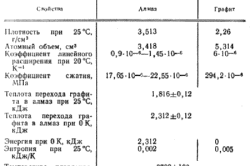
And diamond, and graphite are different forms of the same element - carbon. At the soft, crumbling graphite and the solid crystal in the world in the world, the same formula - C. How is it possible?
Properties of diamond and graphite
Diamonds are found in nature in a well-pronounced crystalline form.It is transparent and most often a colorless crystal, although there are diamonds painted in blue, red and even black colors. Such a color retreat from the rule is associated with the peculiarities of the natural conditions of the formation of the crystal and the presence of impurities in it. The peeled and polished diamond acquires a special shine, which people appreciated.
Diamonds reflect light well and, having a complex form, it is well refracted. This gives genus signs and overflow the purified crystal. It is a heat conductor, but in relation to electricity is an insulator.
 Graphite is an antipode of diamond. This is not a crystal, but a totality of thin plates. He is black with a gray sampling. In appearance resembles steel with the predominance of cast iron.
Graphite is an antipode of diamond. This is not a crystal, but a totality of thin plates. He is black with a gray sampling. In appearance resembles steel with the predominance of cast iron.
Despite the steel appearance, on the touch it is greasy, and when used it turns out to be soft. With the slightest pressure, he crumbles, which attracts a person using graphite as a means of improving information on paper.
Graphite, like a diamond, is a good conductor of heat, but, unlike its fellow on a molecular structure, he also conducts electricity well.
Of these different representatives of the polymorphism of molecular carbon distinguishes from each other only one thing is the structure of the molecular grille. Everything else is only a consequence of the main thing.
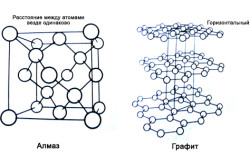
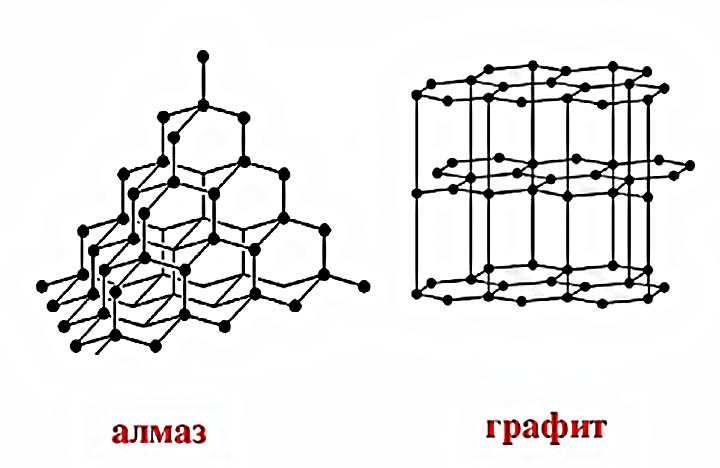
In graphite, the crystal lattice is organized on a plany principle. All its atoms are placed in the hexagon, which are in the same plane. Therefore, the links between the atoms of different hexagons are such fragile, and the graphite itself is layered, and its layers are poorly connected with each other. This structure of the crystal lattice determines its softness and diverse utility, but the graphite itself is destroyed. However, it is the structure of a crystal lattice that allows using special conditions and other substances, to make diamond graphite. The same processes occur with this mineral in nature under similar conditions.
A diamond lattice is built on the principle of volumetric links of all with everyone and all with everyone. Atoms form the correct tetrahedron. Atom in each tetrahedra is surrounded by other atoms, each of which forms the top of the other tetrahedron. It turns out that tetraheders in each piece of diamond are much larger than molecules forming these tetrahedra, since each of the tetrahedra is part of another tetrahedron. For this reason, the diamond is the most indestructible mineral.
Carbon fate in graphite and diamond
 Carbon refers to the most mass elements of the biosphere and the entire planet Earth. It is present in atmosphere in certain states ( carbon dioxide), in water (dissolved carbon dioxide and other connections) and in a lithosphere. Here, in solid terrible, it is part of the large deposits of coal, oil, natural gas, peat, etc. But in its pure form, it is represented by the deposits of diamond and graphite.
Carbon refers to the most mass elements of the biosphere and the entire planet Earth. It is present in atmosphere in certain states ( carbon dioxide), in water (dissolved carbon dioxide and other connections) and in a lithosphere. Here, in solid terrible, it is part of the large deposits of coal, oil, natural gas, peat, etc. But in its pure form, it is represented by the deposits of diamond and graphite.
Most of the carbon is concentrated in living organisms. Any organisms build their body from carbon, the concentration of which in living bodies exceeds the carbon content in inanimate matter. Dead organisms settle on the surface of the lithosphere or ocean. There they decompose in different conditions, forming deposits rich in carbon.
The origin of the pure deposits of diamonds and graphite causes a lot of disputes. There is an opinion that these are former organisms that have fallen into special conditions and mineralized like coal. It is also considered that diamonds have a magmatic origin, and graphite is metamorphic. This means that complex processes in the depths of the earth are involved in the concentration of diamonds on the planet, where spontaneously in the presence of oxygen arises explosion and burning. As a result of the interaction of methane and oxygen molecules and diamond crystals occur. With these processes, but in certain conditions there may be an appearance and graphite.
How to get from diamond graphite
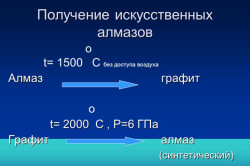 Receipt at the current level of development of chemistry has long been a problem. The fact that nature is done for millions of years, a person can do for much more short term. The main thing is to reproduce the conditions in which in nature one form of pure carbon passed to another, that is, create a high temperature and very high pressure.
Receipt at the current level of development of chemistry has long been a problem. The fact that nature is done for millions of years, a person can do for much more short term. The main thing is to reproduce the conditions in which in nature one form of pure carbon passed to another, that is, create a high temperature and very high pressure.
For the first time, such conditions were created using an explosion. The explosion is instant burning under greater pressure. After you have gathered what we managed to collect, it turned out that small diamonds appeared in graphite. Such a fragmentary transformation occurred because the explosion creates a wide variety of pressure and temperature. Where conditions were created for the transition from graphite in Almaz, this happened.
This volatility of processes made explosions non-prospective for the production of diamonds from graphite. Scientists, however, did not stop, and they continued to expose graphite with all sorts of tests in the hope of making it to become a diamond. The stable result made heating the graphite bar with pulses to a temperature of 2000 ° C, which made it possible to get diamonds of meaningful sizes.
Experiments with high pressure gave unexpected results - graphite turned into a diamond, but when the pressure decreases, it moved into its original state. It is not possible to reduce the distance between carbon atoms only with the help of one pressure. Then they began to combine pressure and high temperature. Finally, it was possible to find out the range of temperature and pressure combinations, in which you can get diamond crystals. True, while only a technical diamond was obtained, the use of which in jewelry was difficult.
 In addition to the high costs of energy provision of the process of translating graphite in Almaz, there was another problem - with an increase in the length of the exposure to the high temperature, graphitization of diamond begins. All these subtleties complicate the industrial production of diamonds. For this reason, in nature, extremely devastating for it remains relevant and profitable.
In addition to the high costs of energy provision of the process of translating graphite in Almaz, there was another problem - with an increase in the length of the exposure to the high temperature, graphitization of diamond begins. All these subtleties complicate the industrial production of diamonds. For this reason, in nature, extremely devastating for it remains relevant and profitable.
To get a diamond designed for jewelry purposes, began to grow crystals using the seed. The finished diamond crystal was exposed to a temperature of 1500 °, which stimulated the growth first quick, and then slow. The greater the crystal, the slower he grew. This effect made interesting experience Only experience, since its production on an industrial scale has become unprofitable. It did not improve the situation and the use of methane as a "feeding" of a growing diamond. At high pressure and temperature, methane is destroyed to carbon and hydrogen. This carbon is "stern" for diamond.
Application of diamond and graphite
Both minerals are widely used in industry.
Diamonds are used:
- in electrical engineering;
- instrument making;
- electronics;
- on drilling plants
- in jewelry.
Graphite is used at:
- production of crucible and other refractory equipment;
- manufacture of lubricants;
- making pencils;
- production of equipment for the electrodesome industry.
Despite the variety of applications of both graphite and diamond in various industries, we can safely talk about the greater benefits of graphite. Diamond because of the ideality of its crystal lattice inert. It can only be used as a diamond. Most of the diamonds mined in nature goes to the needs of the jewelry industry, since the mineral is one of the most expensive gems, becoming a diamond, he stimulates the turnover of money, and this is its basic property in the economy.
Graphite seized from nature becomes not self-sufficient value, but the great worker production. Thanks to its properties, it is also used in its true, natural form, that is, both graphite, and as a means, on the basis of which new substances can be obtained, for example, the same diamond.
Hello, dear readers. As you know, the diamond, despite its fascinating appearance, is a simple matter. In this article, you will learn that the diamond formula is and provides.
The stone behaves in many situations quite non-standard, many experiments and definitions of some values \u200b\u200bdue to this are difficult. However, the properties of the stone are so high that various studies are still carried out, hypotheses are put forward, attempts to create analogues and even substances that are superior to their own diamond properties.
Chemical formula diamond
In fact, everything is very simple:
Diamond Formula - C
This is explained by the fact that the composition of the stone is almost 100% carbon. But the rest of the elements are so small that they are not counted in the formula. In general, in the nature of carbon is not so much - only 0.15% of the total number of items. The sequence number of carbon in Table 6 (i.e. it has 6 protons inside the nucleus). This means that the diamond has the same sequence number (if you once again look at its formula).
Below are given brief characteristics Mineral, many of which depend on the initial chemical formula.
Brief characteristics of diamond and facts about him
- Almaz has, an average estimated as 3.5 g / cm.
- Pure diamond is transparent, but often has colors and shades (rare colors are valued above).
- Very brilliant due to the indicators of dispersion and refraction.
- With all the hardness is very fragile.
- It does a very bad electric current.
- It is possible to transform only graphite into diamond, other allotropic carbon modifications do not succumb. But the reverse reaction is simpler (transformation of the mineral back to graphite), although it happens at much higher temperatures.
- Chemical formula does not affect a significant difference in the properties of varieties of carbon modifications. This is due only to the difference in the structure. crystal lattices substances.
- "Packed" mineral is very tight, has only 18 atoms.
Origin
It is assumed that the creation of a diamond takes a huge amount of time, millions of years, also great pressure and temperature. But it is about natural conditions.
Scientists do not exclude the likelihood of the appearance of stone from outside the Earth orbit. The assumption is based on a large amount of stone in the surrounding outer space. At the same time, the share of carbon itself on Earth is not high.
This hypothesis is also confirmed by the detected varieties of diamond in space meteorites (for example, LonsDelit).
Chemical properties
- Diamond is inherent inertia due to its hardness. In this regard, the burning reaction for the stone is the main:
2c + O2 \u003d 2Co
C + O2 \u003d CO2
- All stone atoms are located so closely from each other. That is, each carbon atom is in the middle of the tetrahedron, and the remaining atoms are located on tops.
- The molar mass is about 12 g / mol.
The video contains the structure of the diamond in the most convenient model. Immediately you can learn about some of the properties of the stone.
Stone use
Stone is widely used in jewelry. But in addition, he finds its use in electronics, optics and even construction. With the help of it, specialized skins are created, they are covered with drills, check metal for strength in installations with diamond tips.
Diamond used in chemical experiments as reliable protection From very caustic reagents like platoric acid. In surgery without a mineral, it is also not to do, because it provides accuracy and miniaturity of cuts. Diamond scalpels are a real find for doctors.
Getting mineral
Now there are many ways to produce stone, as it is more profitable for production than to use natural diamonds. The cost of such stones is also significantly lower. Although properties natural stones What is higher and better, because of which their production does not stop despite the large number of good-quality analogues, including those having a similar composition, but are not diamonds: fullerenes, lansdalet, graphite, carbide and some others.
Also in production, stones with a high content of impurities are sent, which in jewelry will not be useful. Such inclusions should be more than 5% as a whole and more than 2% of one particular substance (they may be calcium, nitrogen, boron and some others). In this case, the appearance of the mineral is very modified and not to fix it.

In nature, the stone is found in the so-called kimblite and lamproit tubes, as well as plants. In the laboratory conditions and production creates a mineral completely differently.
Studies of diamonds and experiments continue with them, since the stone is very promising from the point of view of its properties. Scientists and researchers leave no attempts to find more favorable ways to create artificial stones.
Thus, diamond due to its formula and the structure has a lot of useful propertiesinherent in such ranges only to him. Come on the resource more often and learn more new things about stones and minerals.
Team of loves